U.S. Patent No. 9,675,579 B2 (‘579) issued on June 13, 2017, for “Tetrahydrocannabivarin for use in the treatment of nausea and vomiting.” It was issued to applicant GW Pharma Ltd., of Salisbury, Great Britain, and inventors Erin Rock and Linda Parker of Guelph, Canada, and Marnie Duncan and Collin Stott of Salisbury, Great Britain. The specification discloses background art demonstrating THCV’s effectiveness to suppress seizures, inflammation, pain, and Parkinson’s disease. The disclosure further provides a treatment for using THCV to counter nausea and emesis (vomiting). This is based on THCV’s lack of production of conditioned gaping, which results in compounds SF141716 and AM251, the primary producers of nausea. The claims are directed to the method of treatment using THCV as an anti-nausea and anti-emesis medication. Specific dosing is between 1 – 2000 mg, with a further limitation of 10 – 1000 mg, over a 7 day period. There are numerous specific embodiments also disclosed in the specification.
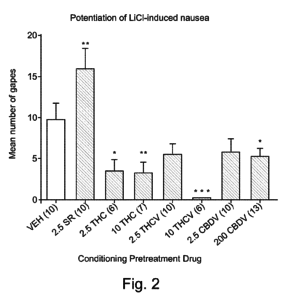
Figure 2 below shows an embodiment demonstrating THCV’s effectiveness at not producing gaping in test trials compared against other cannabis compounds used in the trial.
The International Patent Classification is A61K (preparations for medical, dental or toilet purposes (i.e., methods specially adapted for pharmaceutical products into particular physical form).
GW Pharma has been the market leader on cannabis-based pharmaceutical research. There are almost 100 patents issued to GW Pharma on a variety of cannabis-based drugs, both organic and synthetic uses. More cannabis technology is expected to begin the patenting process as various federal agencies become friendlier to adult-use cannabis, both in research and recreational use.
Please contact Yonaxis for more information on patent processes if you have any questions.