On January 23, 2018, the United States Court of Appeals for the Federal Circuit handed down In re Janssen Biotech, Inc.,[1] which dealt with the specific issue of the double patenting safe harbor under 35 U.S.C. §121.[2] This case is an example of playing games with the patent prosecution system in order to better position the patent for commercial gain may come back to bite you.
The facts of the case are as follows.
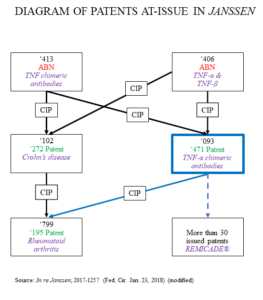
On October 27, 1993, a 5-way restriction requirement was issued during the prosecution of Application Serial No. 08/013,413 (‘413). On February 4, 1994, instead of filing a response, applicant Janssen filed two CIPs, Serial Nos. 08/192,093 (‘093) and 08/192,102 (‘102), and expressly abandoned the ‘413 application. The ‘093 application matured into Patent No. 6,284,471 (‘471), and the ‘102 application matured into Patent No. 5,656,272 (‘272). The ‘093 claimed subject matter from both the ‘413 application and Serial No. 08/010,406 (‘406). ‘413 related to TNF-α antibodies, including chimeric antibodies and its methods, while ‘406 related to immunoreceptors binding to TNF-α and TNF-β. Ten months after filing ‘093, Janssen filed a preliminary amendment amending, cancelling, and adding claims. More importantly, these amendments did not affect ‘093’s CIP status (i.e., ‘093 remained a CIP of both ‘413 and ‘406). In other words, it remained a CIP (although claims no longer claimed subject matter from ‘413) and was not converted into a divisional.
The examiner issued a species requirement,[3] between Species I, chimeric antibodies and immunoreceptors comprising the epitope binding region of an antibody, and Species II, immunoreceptor molecules comprising TNF receptors p55 or p75. Janssen elected Species I. An office action followed, and the examiner provisionally rejected ‘093’s claims on obviousness-type double patenting grounds over Serial No. 08/324,799 (‘799), which is a CIP of ‘102. ‘799 has the same filing date as ‘093, and is also a CIP of both ‘413 and ‘406. Janssen then amended ‘102 and ‘799 through preliminary amendments, cancelling all claims and adding new claims in ‘102 directed to a method to treat Crohn’s disease, and new claims in ‘799 directed to methods of treating rheumatoid arthritis. ‘799 subsequently issued as Patent No. 5,698,195 (‘195).
After the double patenting rejection in ‘093, Janssen cancelled and amended the claims, limiting Claim 1 to TNF-α. ‘093 eventually issued as the ‘471 patent. Claims 1, 3, and 5-6 are directed to TNF-α specific chimeric antibody; claims 2 and 4 are directed to immunoassay methods to detect TNF; and claims 8-9 are directed to polypeptides of amino acid sequences binding to h TNF-α. It should be noted that claim of priority remained a CIP, and not a divisional.
In 2013, an ex parte reexam[4] was filed in ‘471 on double patenting grounds over ‘195 and ‘272. During reexam, Janssen canceled claims 8-9, and requested the CIP priority benefit to ‘406 be deleted. Further, Janssen requested that the full disclosure of ‘471 be conformed to ‘413, and that ‘093 (now ‘471) be designated (retroactively) as a divisional of ‘413.[5] Amendments were entered for procedural reasons, but designation as a divisional was not confirmed. The examiner maintained the double patenting rejections, concluding the 35 U.S.C. §121 safe harbor did not apply. On appeal, the PTAB affirmed, stating that there was “no reason to permit [Janssen] now, by amendment, to acquire the benefit of the safe harbor when [Janssen] voluntarily and deliberately filed a [CIP] with claims directed to subject matter absent from the ‘413 application and outside the scope of its restriction.”[6]
The following diagram visualizes the patents-at-issue in this case:
 The main issue on appeal was whether the safe harbor provision of §121 applied to the ‘471 patent. If so, the referenced patents ‘272 and ‘195 could not be used against the ‘471 patent and invalidate it due to double patenting rejection.
The main issue on appeal was whether the safe harbor provision of §121 applied to the ‘471 patent. If so, the referenced patents ‘272 and ‘195 could not be used against the ‘471 patent and invalidate it due to double patenting rejection.
Our analysis first starts out with what the definitions are for CIP (continuation-in-part) and divisional. The MPEP is instructive. A CIP is an application filed during the lifetime of an earlier application by the same applicant claiming a portion of the earlier application’s subject matter and also included new matter not previously disclosed.[7] A divisional is an application filed during the lifetime of an earlier application by the same applicant and claims a carve-out of that earlier application; it is mostly the product of a restriction requirement.[8] Restriction is the practice of requiring a patent applicant to elect a single claimed invention for examination when two or more independent or distinct inventions are claimed in a single application.[9]
Double patenting is:
A doctrine which seeks to prevent the unjustified extension of patent exclusivity beyond the term of a patent. The public policy behind this doctrine is that:
The public should . . . be able to act on the assumption that upon the expiration of the patent it will be free to use not only the invention claimed in the patent but also modifications or variants which would have been obvious to those of ordinary skill in the art at the time the invention was made, taking into account the skill in the art and prior art other than the invention claimed in the issued patent.[10]
The safe harbor of §121 is:
A patent issuing on an application with respect to which a requirement for restriction under this section has been made, or on an application filed as a result of such a requirement, shall not be used as a reference either in the Patent and Trademark Office or in the courts against a divisional application or against the original application or any patent issued on either of them, if the divisional application is filed before the issuance of the patent on the other application.
(Emphasis added.)[11]
The Fed Circuit panel reasoned that this provision only applies to divisionals and its parent, and does not cover CIPs or continuations.[12] The panel applied a “strict test” to determine whether a reexamination patent can be entitled to protections under the §121 safe harbor. Using Searle as a guidepost, where another Fed Circuit panel found that the §121 safe harbor did not extend to reissues, this panel similarly held that reexams did not, as well.[13] “The doctrine of obviousness-type double patenting is intended to prevent the extension of the term of a patent by prohibiting the issuance of the claims of a second patent that are not patentably distinct from the claims of the first patent.”[14] Further, the panel held, “a patent owner cannot retroactively bring its challenged patent within the scope of the safe-harbor provision by amendment in a reexam proceeding.”[15]
Janssen tried to argue that Searle was inapposite because Janssen did not enjoy a benefit at the public’s expense because no claim as issued in ‘471 relied upon new matter in ‘093. The Fed Circuit panel was not persuaded, stating that Janssen indeed inured a benefit and the public’s expense because more than 30 patents in the patent family that claim priority to ‘471 had been issued. Janssen further argued that there is a “widely followed practice” to file the child divisional application with a copy of the parent application’s specification and claims, along with a preliminary amendment limiting claims to one or more non-elected inventions prior to examination.[16] However, the Fed Circuit panel scoffed at this argument, stating that while this is an MPEP rule, Janssen did not follow it.
The Federal Circuit panel, comprised of Judges Prost, Reyna, and Wallach, affirmed the PTAB’s reexam decision invalidating the ‘471 patent under the doctrine of obviousness-type double patenting.
This case is significant because of the subject matter of this patent family, primarily the TNF-α antibodies and immunoassay methods, are the active compounds in Johnson & Johnson’s REMICADE® drug used to treat a host of auto-immune diseases. Janssen Biotech is a subsidiary of J&J. The ‘471 invalidation may affect the drug’s patent term and bolster its Big Pharma rival Pfizer in pushing for lower cost generics including Pfizer’s own INFLECTRA®.[17]
[1] 880 F.3d 1315 (Fed. Cir. 2018).
[3] See MPEP 806.04(f): “where two or more species are claimed, a requirement for restriction to a single species may be proper if the species are mutually exclusive. Claims to different species are mutually exclusive if one claim recites limitations disclosed for a first species but not a second, while a second claim recites limitations disclosed only for the second species and not the first.”
[4] See 35 USC §302; MPEP 2209 et seq..
[6] Janssen, supra (slip op. at 7-8).
[7] See MPEP 201.08.
[8] See MPEP 201.06.
[9] See MPEP 802.02.
[10] See MPEP 804 (citing In re Zickendraht, 319 F.2d 225, 232, 138 U.S.P.Q. 22, 27 (C.C.P.A. 1963).
[11] See MPEP 802; Janssen, supra (slip op. at 8-9).
[12] Janssen, supra (slip op. at 9) (citing Pfizer, Inc. v. Teva Pharm. USA, Inc., 518 F.3d 1353, 1360 (Fed. Cir. 2008), Amgen, Inc. v. F. Hoffman-La Roche Ltd., 580 F.3d 1340, 1353 (Fed. Cir. 2009)).
[13] See G.D. Searle LLC v. Lupin Pharm., Inc., 790 F.3d 1349, 1354-55 (Fed. Cir. 2015).
[14] Id. at 1351.
[15] Janssen, supra (slip op. at 11).
[16] See MPEP 714.01(e).
[17] See Jan Wolfe, “U.S. appeals court upholds ruling invalidating J&J patent on Remicade,” Reuters (Jan. 23, 2018).