In its second patent-eligibility ruling in a few weeks, the Court of Appeals for the Federal Circuit decided Natural Alts. Int’l, Inc. v. Creative Compounds, LLC,[1] on March 15, 2019, in which it found claims directed to dietary supplements in six patents to be patent-eligible under 35 U.S.C. §101. Natural Alternatives International (NAI) was recently the subject of another Fed Circuit ruling last year dealing with erroneous claims of priority under 35 U.S.C. §120, and unlike that case, which we discussed on the blog, NAI fared better before the Fed Circuit this time around.
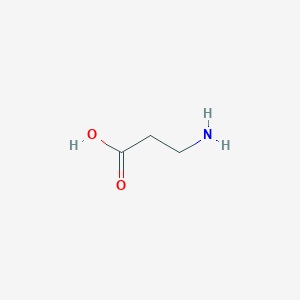
NAI owns the six patents-at-issue – U.S. Patent Nos. 5,965,596, 7,825,084, 7,504,376, 8,993,610, 8,470,865 and RE45,947. All are directed to dietary supplements containing the non-essential amino acid β-alanine. In combination with another amino acid, histidine, the specifications of these six patents teach that with variations in dipeptide concentrations, the dietary compound increases anaerobic capacity, thereby facilitating muscle growth and muscular cell growth necessary for athletic performance.
NAI filed a patent infringement suit against Creative Compounds asserting these six patents. Creative Compounds responded by moving for judgment on the pleadings (F.R.C.P. Rule 12(c)). The motion was granted by the district court, which found the claims directed to naturally occurring phenomena, and therefore, patent-ineligible subject matter under §101.
The Fed Circuit panel was composed of Judges Moore, Reyna, and Wallach, with Judge Moore writing for the court. Judge Reyna wrote an opinion concurring-in-part and dissenting-in-part.
Claims of the six patents-at-issue are directed to methods, products, and manufacturing of β-alanine as a treatment for muscular fatigue. Judge Moore first discussed the method claims. She noted that the recently-decided Vanda case held that claims directed to methods of treating patients were patent-eligible in spite of comprising known naturally occurring relationships between a natural compound through a naturally-occurring process.[2]
[T]he claims were directed to a patent-eligible method of using iloperidone to treat schizophrenia, “a specific method of treatment for specific patients using a specific compound at specific doses to achieve a specific outcome.” [Similarly, t]he Method Claims are directed to patent eligible new ways of using an existing product, beta-alanine . . . [which] falls clearly within the scope of §101.
She buttressed her contention that the NAI Method Claims were patent-eligible based on support in the specifications that contain specific dosing limitations, similar to the Vanda specification. Then, specifically dealing within the context of the Rule 12(c) motion to dismiss, Judge Moore noted:
[The specification] does not establish that the dietary supplement in the claims, which provides a dose well in excess of the normal levels of beta-alanine, would have been well-understood, routine, and conventional. While a fact-finder may ultimately determine that the dietary supplement limitation was well-understood, routine, and conventional, absent a clear statement to that effect in the specification, complaint, or other material properly before the court, when disputed such a determination may not be made on a motion for judgment on the pleadings.
As to the Product Claims, Judge Moore found that β-alanine is a natural product, the Product Claims themselves are not directed to β-alanine itself. Rather, the Product Claims are directed to specific treatment formulations that, nevertheless, are composed of natural products (i.e., β-alanine and the other amino acids and natural substances), but that they also contain different characteristics and uses – such characteristics and uses which can be used for treatment that β-alanine alone in nature cannot do. Specifically, β-alanine, in combination with other amino acids and in specific dosing administrations, are used to increase athletic performance. She continued to rationalize β-alanine by itself in nature cannot increase athletic performance. Therefore, the Product Claims are patent-eligible under §101.
Finally, as to the Manufacturing Claims, she posited:
Claim 1 of the ‘610 patent is even further removed from the natural law and product of nature at issue in the Method Claims and Product Claims, respectively. It is directed to the manufacture of a human dietary supplement with certain characteristics. The supplement is not a product of nature and the use of the supplement to achieve a given result is not directed to a law of nature. We do not see, therefore, how a claim to the manufacture of a non-natural supplement would be directed to the law of nature or natural product.
Judge Reyna concurred-in-part and dissented-in-part. He concurred only in the remand for further proceedings. As to the dissent, Judge Reyna took issue with the majority’s reliance on what he found to be erroneous claim construction. Because of the procedural mechanism used to bring the case to the appellate level, he believed there was insufficient opportunity by the district court to properly conduct claim construction. Therefore, the majority relies solely on NAI’s construction of the claim terms, which were adopted by the district court. He believed NAI’s construction of “human dietary supplement” and “dietary supplement” improperly imported limitations into the claims, and incorporated definitions that were contrary to the plain meaning requirement of the Phillips standard. As such, Judge Reyna would have remanded the case back to the district court for a ruling on the substantive §101 issues after it had properly conducted a Markman-style claim construction.
Natural Alternatives will probably be one of the more important cases in the §101 jurisprudence because of the fine-detail delineation between eligible treatment claims and ineligible natural products. As a final recitation, Judge Moore noted in her opinion:
We live in the natural world, and all inventions are constrained by the laws of nature. As the Supreme Court has warned, we must be careful not to overly abstract claims when performing the Alice analysis.
[1] ___F.3d___ (Fed. Cir. 2019), reversed and remanded, Case No. 3:16-cv-02146-H-AGS, Order Granting Defendant’s Motion for Judgment on the Pleadings (S.D. Cal. Sept. 5, 2017).
[2] See Vanda Pharms Inc. v. West-Ward Pharms. Int’l Ltd., 887 F.3d 1117, 1134-36 (Fed. Cir. 2018).