U.S. Patent No. 10,265,362 B2 (‘362) issued on April 23, 2019, for “Ingestible Films Having Substances From Hemp or Cannabis.” It was issued to applicant/inventor Scott Schaneville, of St. Petersburg, Florida.
Figure 1-2 illustrates the claimed invention:
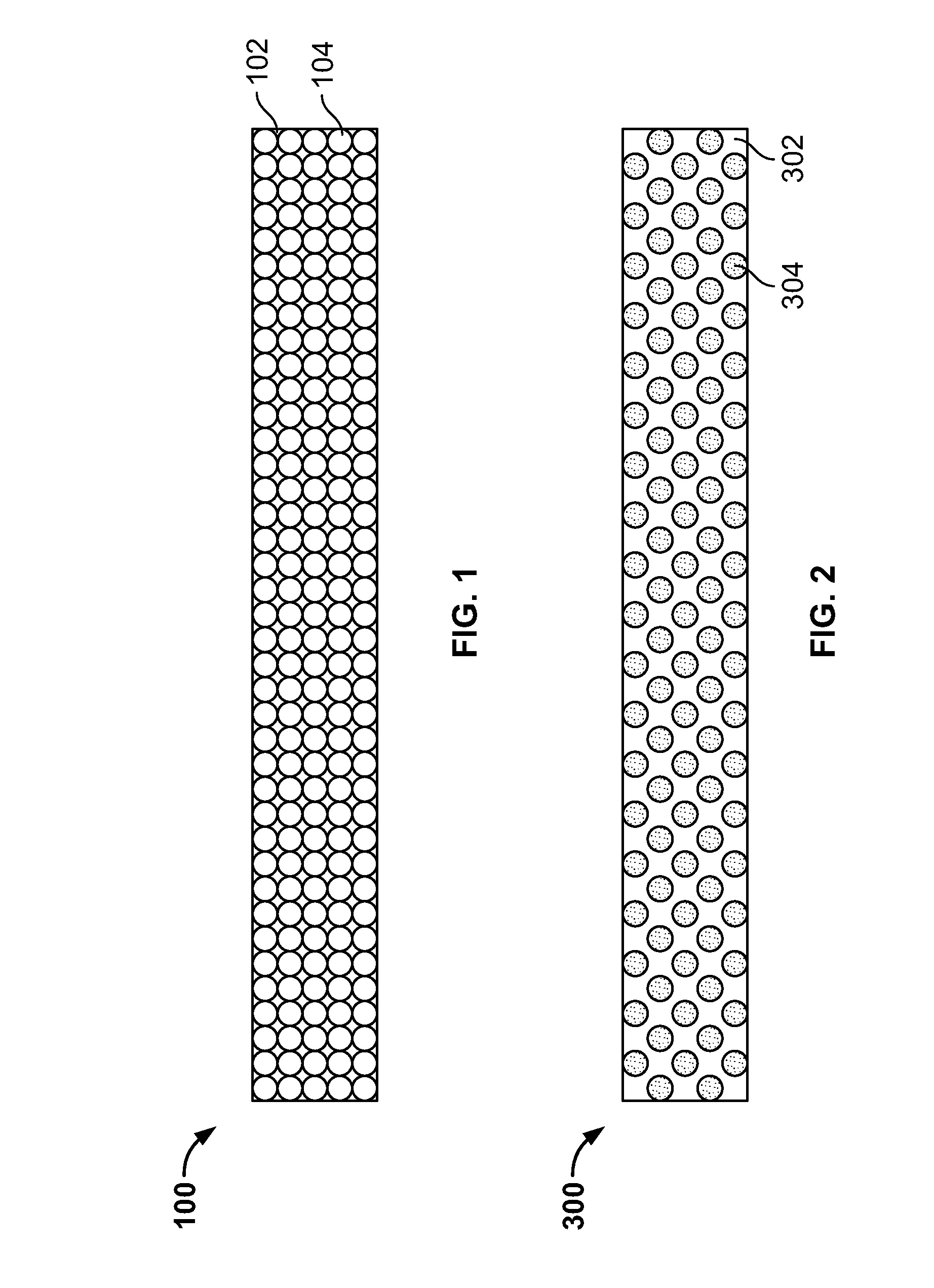
The ‘362 patent discloses a new system for cannabis medical drug delivery, specifically through mucosal oral administration of a highly dissolvable, improved taste, and non-sticky wafer containing cannabidiol (CBD) or tetrahydrocannabinol (THC) in concentration of 90 – 100%. The novelty (35 U.S.C. §102) of the invention is through its improved taste, mucosal administration and high CBD/TCH concentrations for faster therapeutic and medical benefits. The claims are directed to that composition comprising the film including cannabis extracts (CBD, THC), with a sucrose ingredient, like honey or sugar, pectin, polysorbate, and guar gum, among others.
The Cooperative Patent Classification is A61K (preparations for medical, dental, or toilet purposes, namely, medicinal preparations or undetermined constitution containing traditional herbal medicines, namely, magnoliopsida (36/185), an organic active ingredient, namely, condensed with carbocyclic rings, e.g., cannabinols (31/352), targeting or modifying agents chemically bound to the active ingredient, namely, polysaccharides or derivatives thereof (47/36) and carbohydrates, e.g., sugar alcohols, amino sugars, and saccharides (47/26), and special physical form, namely, oral mucosa, e.g., mucoadhesive forms, subligual droplet, or buccal patches or films (9/00, 9/06)).
Please contact Yonaxis for more information on any of the IP processes described in this posting if you have any questions.
