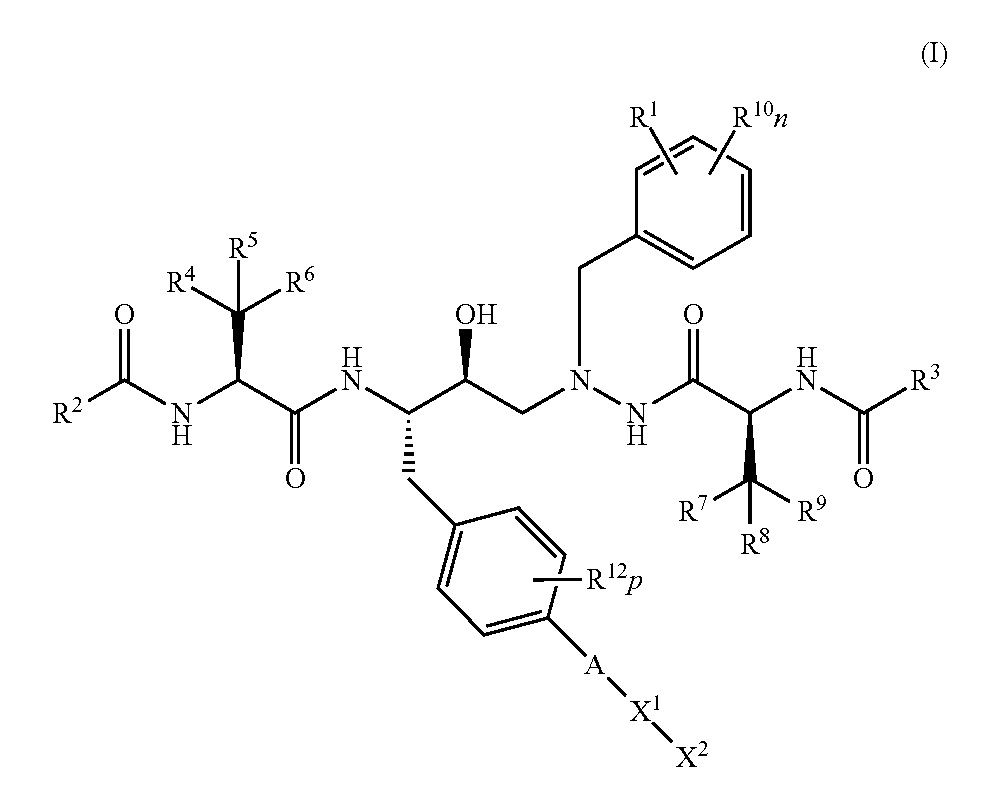
U.S. Patent No. 10,752,636 B2 (‘636) issued on August 25, 2020, entitled ”HIV Inhibitor Compounds.” It was issued to inventors Elizabeth Bacon, Elbert Chin, Jeromy Cottell, Ashley Katana, Darryle Kato, John Link, Nathan Shapiro, Teresa Trejo Martin, and Zheng-Yu Yang. The applicant/assignee is Gilead Sciences, Inc., of Foster City, California. The ‘636 patent is a family member related to Gilead’s HIV drugs, TRUVADA® and DESCOVY®.
The compound including emtricitabine and tenofovir diproxil fumarate is marketed and distributed under the trade name TRUVADA®, registered as a trademark in International Class 5, for pharmaceutical preparations for the treatment of infectious conditions.
The compound including emtricitabine and tenofovir diproxil alafenamide is marketed and distributed under the trade name DESCOVY®, registered as a trademark in International Class 5, for pharmaceutical preparations for the prevention and treatment of hepatitis and HIV infection; antivirals; anti-inflammatories; anti-fungals, and pharmaceutical preparations for use in the treatment of infectious diseases.
The Cooperative Patent Classification is C07D, heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed bridged system (487/08), containing three or more hetero rings (405/14), ortho-condensed systems (491/04), and linked by a chain containing hetero atoms as chain links (403/12); and A61K, preparations for medical, dental, or toilet purposes, namely heterocyclic compounds having nitrogen as a ring hetero atom, with five-membered rings, e.g., purines (31/52), and having seven-membered rings (31/55) having at least one nitrogen and one oxygen as ring hetero atoms (31/553).
Gilead’s Truvada and Descovy have been used in pre-exposure prophylaxis (PrEP) for HIV prevention since 2004. However, controversy over the costs to patients has proliferated over the last decade. The controversy stems in part because the first patent in the Truvada patent family was filed and issued to a group of National Institutes of Health (NIH) researchers as inventors, which means taxpayers paid for the research and patent costs; the patent was then exclusively licensed to Gilead for distribution of the drug. The Department of Health and Human Services filed a patent infringement suit in district court in Delaware, prompting Gilead to petition the USPTO’s PTAB for institution of an inter partes review of four of the HHS’s patents related to Truvada and Descovy. The petition to institute was denied by the PTAB earlier in 2020, but it does not signal the end of the litigation between the HHS and Gilead over the drug costs.
Any further developments will be discussed on the blog.