U.S. Patent No. 10,695,361 B2
Given the current coronavirus pandemic, much has been discussed about possible vaccine trials and antiviral treatments to coronavirus. One patent directed to therapeutic use is U.S. Patent No. 10,695,361 B2 (‘361) issued on June 30, 2020, entitled ”Methods for Treating Arenaviridae and Coronaviridae Virus Infections.” It was issued to inventors Michael O’Neil Hanrahan Clarke, Joy Yang Feng, Robert Jordan, Richard L. Mackman, Adrian S. Ray, and Dustin Siegel. The applicant/assignee is Gilead Sciences, Inc., of Foster City, California. The ‘361 patent is a family member related to Gilead’s antiviral drug remdesivir.
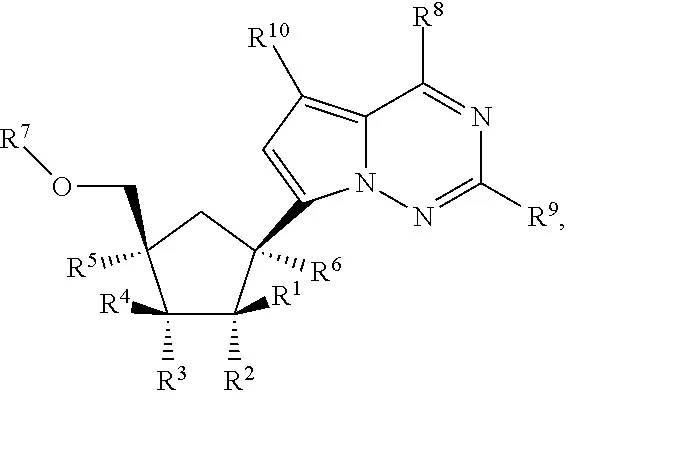 Chemical structure for remdesivir
Chemical structure for remdesivir
The specification discloses numerous chemical structural embodiments in treatment of both arena viruses (e.g., Junin virus, Lassa virus) and corona viruses (e.g., MERS, SARS). The ‘361 claims are directed to the compounds for treatment of specifically coronaviruses and includes the Formula IV compound, which is the structure for remdesivir.
Further, remdesivir is marketed and distributed under the trade name VEKLURY®, which is registered as a trademark in International Class 5, for pharmaceutical preparations for the treatment of viral infections, among others.
The Cooperative Patent Classification is A61K, preparations for medical, dental, or toilet purposes, namely carbohydrates, sugars, and derivatives thereof containing five-membered (31/7056) and six-membered (31/706) rings with nitrogen as a ring hetero atom.
While there has been a lot reported on the use of remdesivir, a drug initially invented for treatment of Ebola virus. However, studies have been mixed, from useful in reducing lower respiratory tract infections resulting from COVID-19 (Beigel study), to inconclusive in a longer-term treatment compared against standard care (Spinner study). While more studies and trials are needed, the patents underlying these new treatments serve as a necessary component in drug development.

Brent T. Yonehara
Founder & Patent Attorney
Founder Brent Yonehara brings over 20 years of strategic intellectual property experience to every client engagement. His distinguished career spans AmLaw 100 firms, specialized boutique I.P. practices, cutting-edge technology companies, and leading research universities.
More About Brent