This case, Vanda Pharmaceuticals Inc. v. West-Ward Pharmaceuticals Int’l Ltd.,[1] arrived to the appeals court through an unusual, but not uncommon, route: a 35 U.S.C. §271(e)(4)(A) infringement resulting from a 21 U.S.C. §355(j) application, or the Abbreviated New Drug Application (ANDA) (aka, generic drug approval process). However, the other aspect of this case is that West-Ward argued Vanda’s patent was invalid under 35 U.S.C. §101 as unpatentable subject matter. Because the Fed Circuit found the claims patent-eligible subject matter, Vanda has the potential to become a major case in the §101 case library.
The facts are as follows.
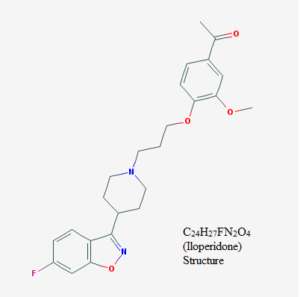
U.S. Patent No. 8,586,610 (‘610) relates to a method for treating schizophrenia with iloperidone using individualized dosages based on the patient’s genotype. The enzyme in the CYP2D6 gene metabolizes many drugs, including iloperidone. Treatment involves increasing this gene in a schizophrenic patient, who typically have lower CYP2D6 activity. Because of the risk of cardiac issues related to the natural interaction between CYP2D6 and the QT prolongation, specialized doses would help address this problem. QT refers to the interval between the Q and T waves of the heart rhythm. The corrected based on the patient’s heart rate it is “QTc.” Vanda used FANAPT® as the trade name for iloperidone in marketing the drug.
Claim 1 was deemed representative:
A method for treating a patient with iloperidone, wherein the patient is suffering from schizophrenia, the method comprising the steps of:
determining whether the patient is a CYP2D6 poor metabolizer by:
obtaining or having obtained a biological sample from the patient;
and
performing or having performed a genotyping assay on the biological sample to determine if the patient has a CYP2D6 poor metabolizer genotype; and
if the patient does not have a CYP2D6 poor metabolizer genotype, then internally administering iloperidone to the patient in an amount that is greater than 12 mg/day, up to 24 mg/day,
wherein a risk of QTc prolongation for a patient having a CYP2D6 poor metabolizer genotype is lower following the internal administration of 12 mg/day or less than it would be if the iloperidone were administered in an amount of greater than 12 mg/day, up to 24 mg/day.
West-Ward filed its ANDA application in 2013, and certified that Vanda’s patents were invalid and/or would not be infringed by West-Ward. West-Ward’s proposed label for its iloperidone generic drug was substantially identical to the FANAPT® label. The district court, sitting in the District of Delaware, in a bench trial found that West-Ward liable for induced infringement under §271(b).
The more important argument West-Ward argued was that the ‘610 patent was invalid under §101 as patent-ineligible subject matter. Patent-eligibility begins with Alice test; first, whether the claims are directed to a patent-ineligible concept. Second, if so, then the claim elements are examined if it contains an inventive concept that will transform the patent into “significantly more” than the abstract idea itself.[2] The panel, consisting of Judges Prost, Lourie, and Hughes, with Judge Lourie writing for the majority, found that claim 1 was patent-eligible. While West-Ward argued that claim 1 was the same as claim 1 in Mayo,[3] the majority disagreed:
This case [] is not Mayo. First, the claims in Mayo were not directed to a novel method of treating a disease. Instead, the claims were directed to a diagnostic method based on the relationships between concentrations of certain metabolites in the blood and the likelihood that a dosage of a thiopurine drug will prove ineffective or cause harm. This relation is a consequence of the ways in which thiopurine compounds are metabolized by the body – entirely natural processes. And so a patent that simply describes that relation sets forth a natural law.[4]
Judge Lourie distinguished the Mayo claims, which were directed to administering a drug, rather than treating a disease; this is unlike the ‘610 patent claim 1 which is directed to a method of using iloperidone to treat a disease, as here, schizophrenia. The ‘610 patent further distinguishes from Mayo in that a physician must administer specific dosages of iloperidone to the patient, depending on the genotype assay results. Judge Lourie continued with the distinction between Mayo and Vanda claim 1, noting that Mayo did not go beyond recognizing (i.e., “indicating,” or “determining”), whereas Vanda actually recite steps to carry out a drug dosage regimen.
Further, he cited CellzDirect for patent-eligibility. In CellzDirect,[5] method claims directed to preparation of cryopreserved hepatocyte cells was patent-eligible subject matter. The claims were not just observing or detecting hepatocytes to survive in deep freeze cycles, but rather preserving hepatocyte cells in a new and useful method of preservation of those cells.[6] The majority affirmed the district court finding, and further affirmed injunctive relief under §271(e)(4).
Chief Judge Prost dissented. She asserted that the claims were directed to a law of nature, and were nothing more than “an optimization of an existing treatment of schizophrenia, just as the Mayo claims concerned ‘optimizing therapeutic efficacy’ of thiopurine drugs.”[7]
This is really a major case in the §101 athenaeum. The majority has made a point when it distinguishes Mayo. Mayo is another personalized medicine patent case, where the mere measurement of the drug administration of metabolites, and then determining the appropriate dosage of that drug based on the measurement was not patent-eligible subject matter according to the U.S. Supreme Court. Measuring and determining is just data-gathering, which is well-understood, routine or conventional.[8] Placed side-by-side, the claims 1 of both cases are be seen below:
| Vanda Pharm., Claim 1 | Mayo, Claim 1 |
|
A method for treating a patient with iloperidone, wherein the patient is suffering from schizophrenia, the method comprising the steps of: determining whether the patient is a CYP2D6 poor metabolizer by: obtaining or having obtained a biological performing or having performed a if the patient does not have a CYP2D6 wherein a risk of QTc prolongation for a patient having a CYP2D6 poor metabolizer genotype is lower following the internal administration of 12 mg/day or less than it would be if the iloperidone were administered in an amount of greater than 12 mg/day, up to 24 mg/day. |
A method of optimizing therapeutic efficacy for treatment of an immune-mediated gastrointestinal disorder, comprising: (a) administering a drug providing 6-thioguanine to a subject having said immune-mediated gastrointestinal disorder; and (b) determining the level of 6-thioguanine in said subject having said immune-mediated gastrointestinal disorder, wherein the level of 6-thioguanine less than about 230 pmol per 8×108 red blood cells indicates a need to increase the amount of said drug subsequently administered to said subject and wherein the level of 6-thioguanine greater than about 400 pmol per 8×108 red blood cells indicates a need to decrease the amount of said drug subsequently administered to said subject. |
As for the wording between the two claim sets, the preambles are different. Mayo’s preamble recites a “method of optimizing therapeutic efficacy,” or just a conventional method. Vanda’s preamble focuses on a “method for treating a patient,” which is a very subtle distinction – it focuses on “something more” than just the abstraction of treatment optimization itself. Further, while Mayo merely “determines” the dosage, Vanda “determines” it and also includes the extra step to make adjustments depending on the individualized genotype assay.
This is further distinguished from another medical diagnostics case, Ariosa, where a naturally-occurring phenomenon (i.e., fetal cffDNA present in blood serum) which was discovered and used in mere conventional testing methods was found not to be patent-eligible subject matter.[9]
This is now the fifth precedential §101 case issued by the Fed Circuit this year alone; further, it is also the fifth case which had at least one claim found patent-eligible. So, patent-eligibility is five-for-five at the Fed Circuit, which has not happened since the Mayo/Alice enunciations in 2014. This is important for the stability of patent law and consistency in the patent practice; both are essential in repairing what many see as a broken patent system. What started as hope with Finjan earlier this year has culminated to the present Vanda, which is making for an exciting year for §101 jurisprudence.
[1] __F.3d___ (Fed. Cir. 2018), aff’g Vanda Pharm. Inc. v. Roxane Labs, Inc., 203 F. Supp. 3d 412 (D. Del. 2016).
[2] Id. (slip op. at 6-7). See also Alice Corp. Pty. v. CLS Bank Int’l, 134 S. Ct. 2347, 2354-55 (2014).
[3] See Mayo Collaborative Services v. Prometheus Laboratories, Inc., 566 U.S. 66, 74-75, 132 S. Ct. 1289 (2012).
[4] See Vanda Pharm., supra (slip op. at 29).
[5] See Rapid Litig. Mgmt. Ltd. v. CellzDirect, Inc., 827 F.3d 1042, 1047 (Fed. Cir. 2016).
[6] Id. at 1048.
[7] See Vanda Pharm., supra (slip op. at 5) (Prost, C.J., dissenting).
[8] See Mayo, supra at 86-87.
[9] Ariosa Diagnostics, Inc. v. Sequenom, Inc., 788 F.3d 1371, 1378 (Fed. Cir. 2015), reh’g en banc denied, 809 F.3d 1282, 1287 (Fed. Cir. 2015), cert. denied, Sequenom, Inc. v. Ariosa Diagnostics, Inc., 136 S. Ct. 2511 (2016).
